Knock-in
对于Knock-in,目前还存在一定的难度,大多数的出版物只能实现10-40%的敲入效率,不同位点也存在差异。Nature Biotechnology今年5月的一篇出版物利用独有的SLEEK (SeLection by Essential-gene Exon Knock-in)技术可以在T细胞上实现超过90%的KI效率,同样在其他常用的免疫细胞如NK,B cell,IPSC等细胞都实现了非常高的KI效率。
对于常规的KI实验,我们依然可以从以下方面考量进行进一步优化:
Donor DNA用量的测试
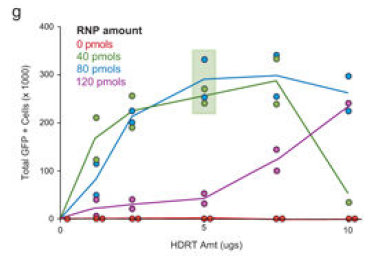
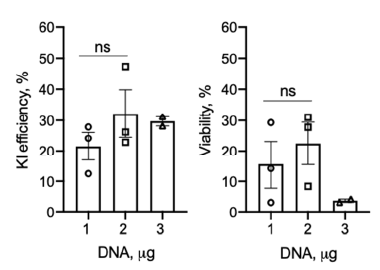
▲ Theodore L Roth et al., 2018 ▲ Zelda Odé et al., 2020
HA长度
HA的长度会影响KI的效率,但并不意味着越长越好。
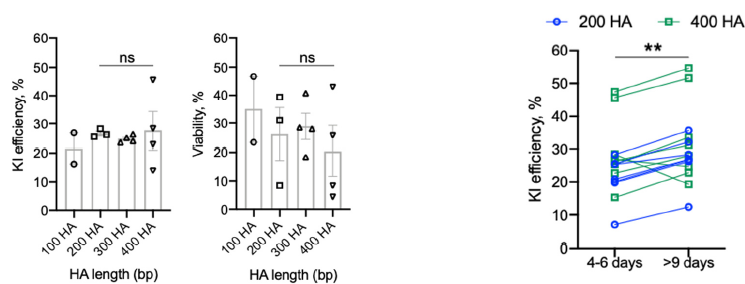
▲ Zelda Odé et al., 2020
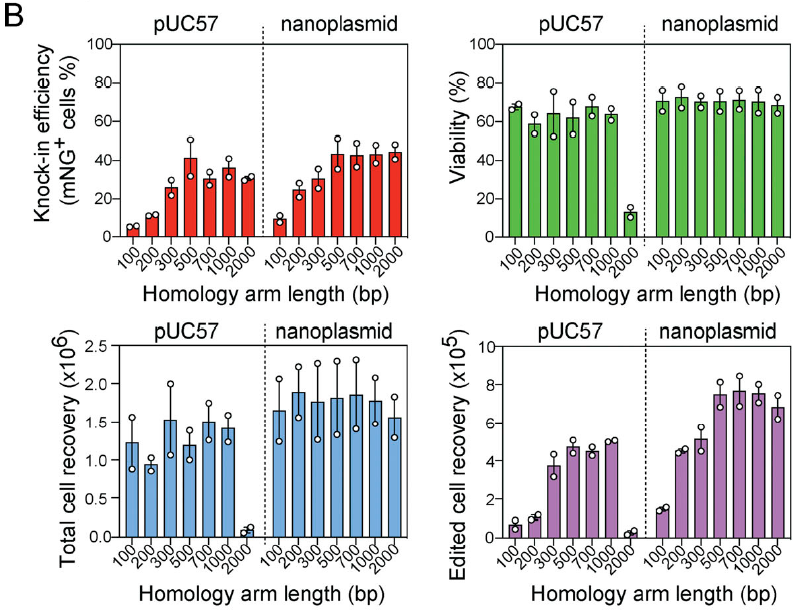
▲ Soyoung A. Oh et al., 2022
HA修饰
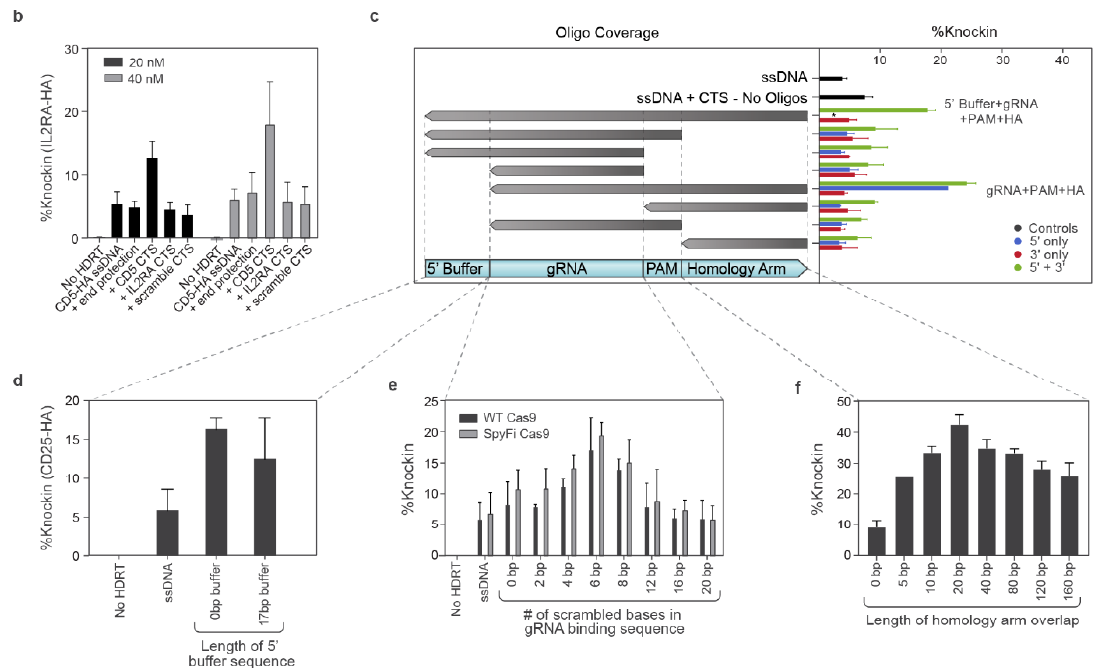
▲ Brian R. Shy et al., 2021

▲ Jonas Kath et al., 2022
特殊成分提高HDR效率的测试
也有文献结果表明PGA对KI的效率没有帮助,仅供参考。
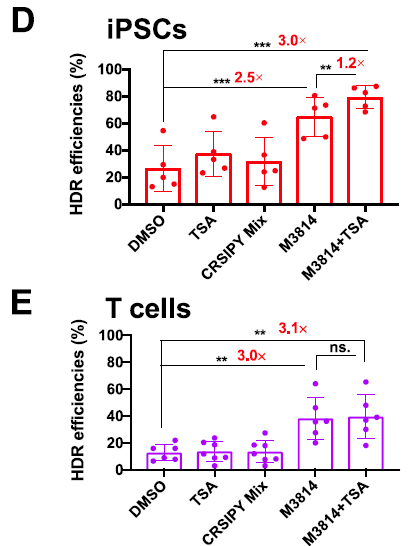
▲ Ya-Wen Fu et al., 2021
▲ David N. Nguyen et al., 2020
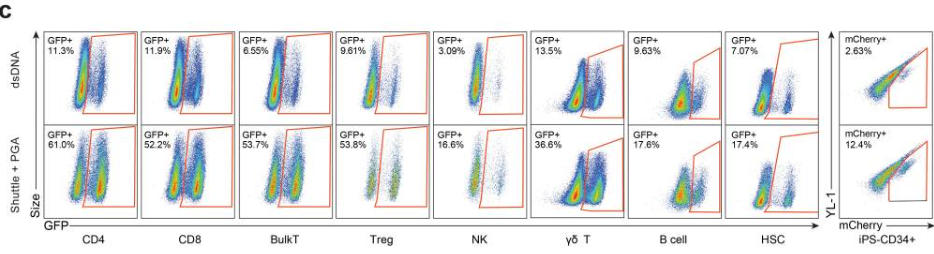
选择dsDNA或ssDNA或AAV载体进行KI
dsDNA会随着用量的增加显著增加对细胞活率的影响。
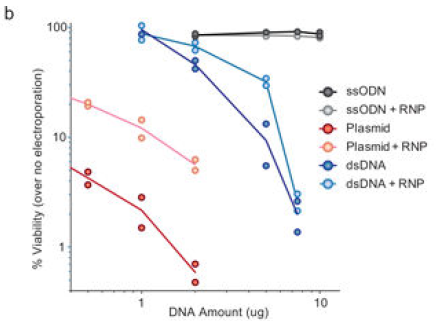
▲ Theodore L Roth et al., 2018
HDR Enhancer

▲ Jonas Kath et al., 2022
甚至HDR, RNP, 细胞的混匀顺序也可能会对结果造成影响
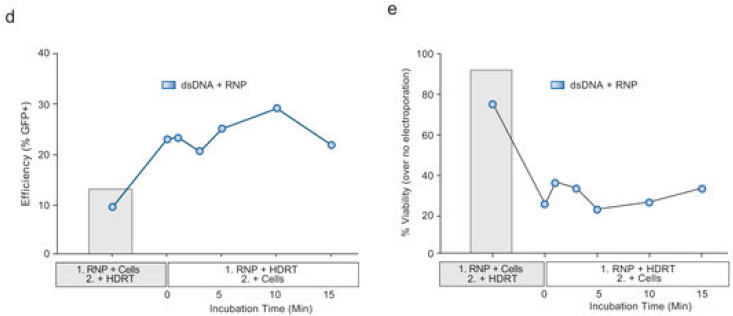
▲ Theodore L Roth et al., 2018
电转前后培养体系
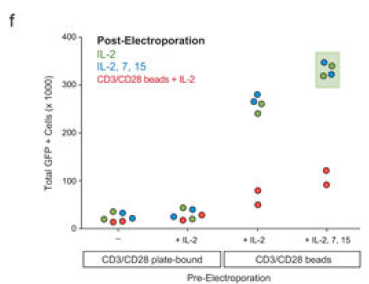
▲ Theodore L Roth et al., 2018
Nucleofection 条件优化
通常可以参考KO的条件进行KI实验,但依然可以依照具体的实验需求调整。
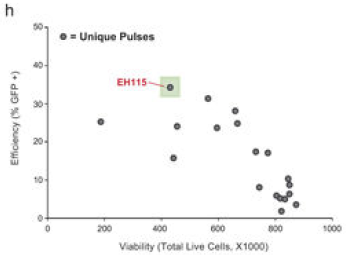
▲ Theodore L Roth et al., 2018

▲ Soyoung A. Oh et al., 2022
对于Knock-in,还可以通过其他手段提升结果,如PAM and Track mutations, 具体内容可以参考如Cas蛋白或sgRNA供应商。
通常KO不会对细胞的活率产生太大影响,但是对于KI,需要用到donor DNA,如dsDNA,或者使用更强的电转染条件,这些可能会导致活率的负面影响。
[参考文献]
1. Akiko Seki et al., Optimized RNP transfection for highly efficient CRI SPR/Cas9-mediated gene knockout in primary T cells. J Exp Med. 2018 Mar 5;215(3):985-997. doi: 10.1084/jem.20171626.
2. Liyang Zhang et al., AsCas12a ultra nuclease facilitates the rapid generation of therapeutic cell medicines. Nat Commun. 2021 Jun 23;12(1):3908. doi: 10.1038/s41467-021-24017-8.
3. Matteo Martufi et al., Single-Step, High-Efficiency CRISPR-Cas9 Genome Editing in Primary Human Disease-Derived Fibroblasts. CRISPR J. 2019 Feb;2(1):31-40. doi: 10.1089/crispr.2018.0047.
4. Alexander G. Allen et al., A highly efficient transgene knock-in technology in clinically relevant cell types. Nat Biotechnol. 2023 May 1. doi: 10.1038/s41587-023-01779-8.
5. Theodore L Roth et al., Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018 Jul;559(7714):405-409. doi: 10.1038/s41586-018-0326-5.
6. Zelda Odé et al., CRISPR-Mediated Non-Viral Site-Specific Gene Integration and Expression in T Cells: Protocol and Application for T-Cell Therapy. Cancers (Basel). 2020 Jun 26;12(6):1704. doi: 10.3390/cancers12061704.
7. Soyoung A. Oh et al., High-efficiency nonviral CRISPR/Cas9-mediated gene editing of human T cells using plasmid donor DNA. J Exp Med. 2022 May 2;219(5):e20211530. doi: 10.1084/jem.20211530.
8. Brian R. Shy et al., Hybrid ssDNA repair templates enable high yield genome engineering in primary cells for disease modeling and cell therapy manufacturing. doi: https://doi.org/10.1101/2021.09.02.458799
9. Jonas Kath et al., Pharmacological interventions enhance virus-free generation of TRAC-replaced CAR T cells. Mol Ther Methods Clin Dev. 2022 Apr 12;25:311-330. doi: 10.1016/j.omtm.2022.03.018.
10. Ya-Wen Fu et al., Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res . 2021 Jan 25;49(2):969-985. doi: 10.1093/nar/gkaa1251.
David N. Nguyen et al., Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat Biotechnol. 2020 Jan;38(1):44-49. doi: 10.1038/s41587-019-0325-6.
来源于优宁维药物研发官网