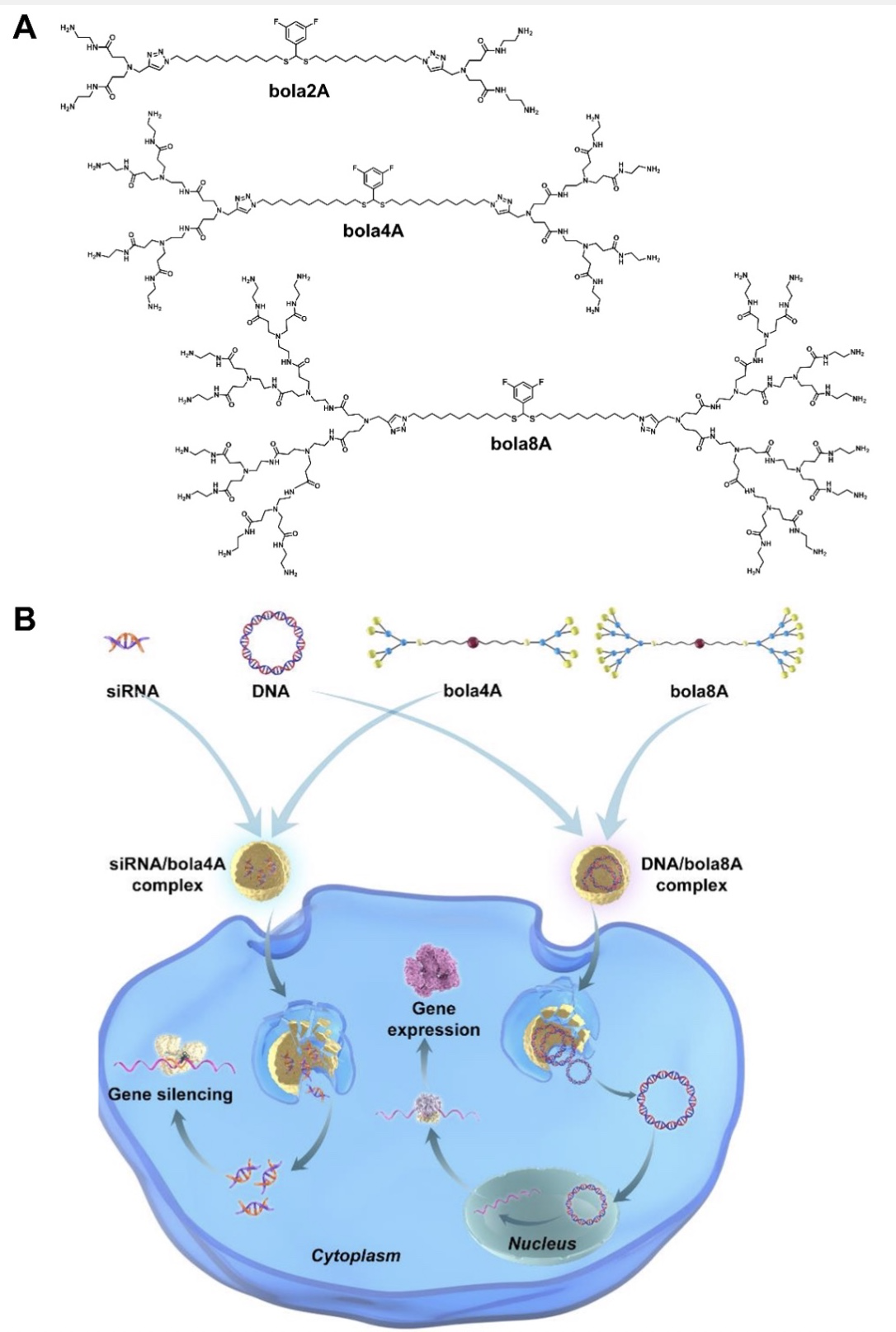
Fig. 1. Bola-amphiphilic dendrimers for cargo-specific nucleic acid delivery. (A) Chemical structures of the bola-amphiphilic dendrimers bola2A, bola4A, and bola8A studied in this work. (B) Cartoon illustration of bola-amphiphilic dendrimers bola4A and bola8A for cargo-selective and adaptive delivery of the two distinct nucleic acid types, DNA and siRNA.
合作团队设计并合成了不同代数的bola型两亲性树形分子并对其递送不同核酸分子的能力进行了深入研究。结果表明,bola树形分子的代数、核酸分子的大小以及bola树形分子与核酸分子之间多价协同作用在很大程度上影响其选择性递送核酸分子的能力。例如,更高代数的bola8A能更有效地将大尺寸质粒DNA 压缩成小的纳米复合物,显著提高了复合物在靶细胞的摄取,展现出更为优异的DNA转染活性;较低代数的bola4A则在有效保护短链siRNA分子的同时,能更高效地促进siRNA 的胞内释放,进而发挥强有力的基因沉默效果(Figure 2)。此外, 该类bola两亲性树形分子所构建的核酸药物递送系统能选择性富集在肿瘤组织,并在肿瘤细胞内ROS刺激下特异性地释放核酸,实现核酸药物在肿瘤细胞的靶向递送。更令人欣喜的是,上述递送体系在宫颈癌和卵巢癌异种移植小鼠肿瘤模型、以及高侵袭性的黑色素瘤和三阴性乳腺癌转移模型中,均表现出可媲美商业化载体的核酸递送效率,能够精准调控致病基因的表达,发挥高效的抗肿瘤活性和抗肿瘤转移效果(Figure 3)。
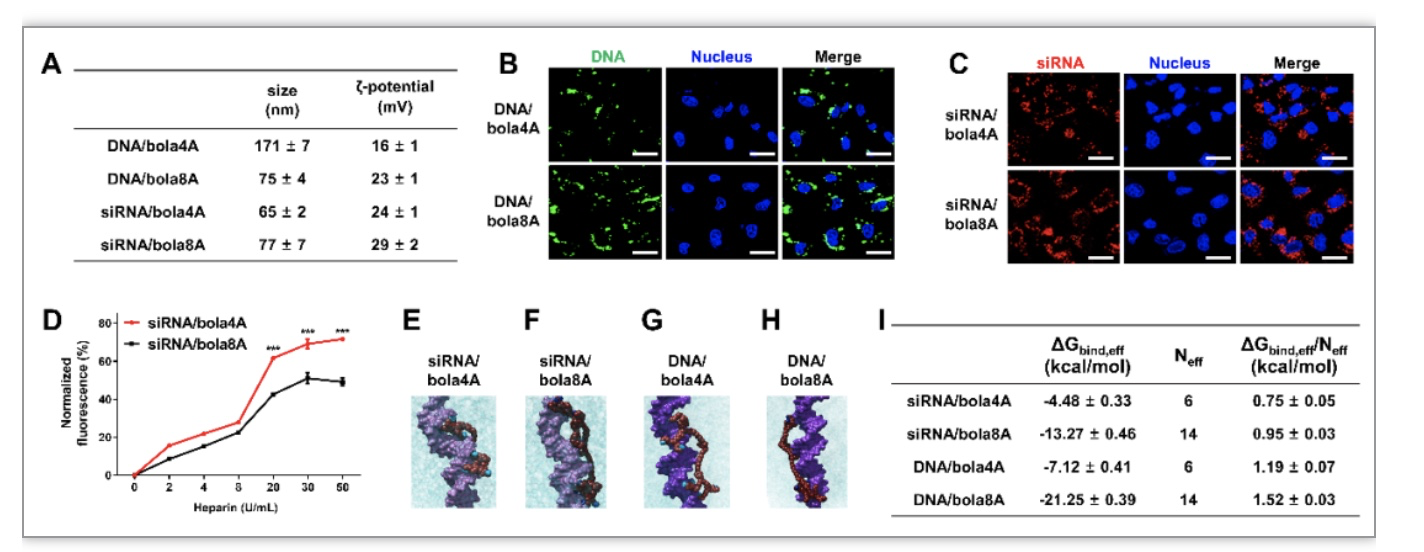
Fig. 2. Physicochemical characterization of and rationale behind the cargo-selective delivery performance of bola4A and bola8A. (A) The sizes and the ζ-potentials of the DNA/dendrimer complex and the siRNA/dendrimer complex obtained with DNA (24 ng/μL) at an N/P ratio of 2 and siRNA (1.0 μM) at an N/P ratio of 10. (B, C) Cellular uptake and intracellular trafficking of DNA and siRNA delivered by the bola-amphiphilic dendrimers bola4A and bola8A. Confocal imaging of the cellular uptake of (B) the DNA/dendrimer complexes (12 ng/μL YOYO-1-labeled DNA, N/P ratio of 1.0) and (C) the siRNA/dendrimer complexes (50 nM Cy5-labeled siRNA, N/P ratio of 10) in SKOV-3 cells, evaluated using confocal microscopy. The green channel image shows the YOYO-1-labeled DNA (green), the red channel image shows the Cy5-labeled siRNA (red), and the blue channel image shows the nuclei of the SKOV-3 cells stained by Hoechst33342 (blue). (D) The siRNA release from the siRNA/dendrimer complexes assessed using heparin-coupled ethidium bromide (EB) fluorescence assays. ***p < 0.001 versus siRNA/bola4A or siRNA/bola8A, and significance was determined using two-way ANOVA (mean ± SD, n = 3). Atomistic MD simulations of bola4A and bola8A in the presence of siRNA (E and F) and DNA (G and H), respectively. Bola4A atoms are shown as “firebrick spheres”, with the terminal charged amine groups highlighted in deep sky-blue, while bola8A atoms are depicted as dark red spheres with the terminal charged amines colored in navy blue. The siRNA (“orchid” light purple) and the DNA (dark purple) are portrayed as their van der Waals surface and the oxygen atoms in water are shown as cyan transparent spheres. Hydrogen atoms, ions and counterions (Na+ and Cl-) are omitted for clarity. (I) Binding data of bola4A and bola8A with siRNA and DNA as derived from atomistic MD simulations: free energy of effective binding (ΔGbind,eff), number of effective charges (Neff), and effective-charge-normalized free energy of binding (ΔGbind,eff/Neff) for the nucleic acid/dendrimer complexes are listed.
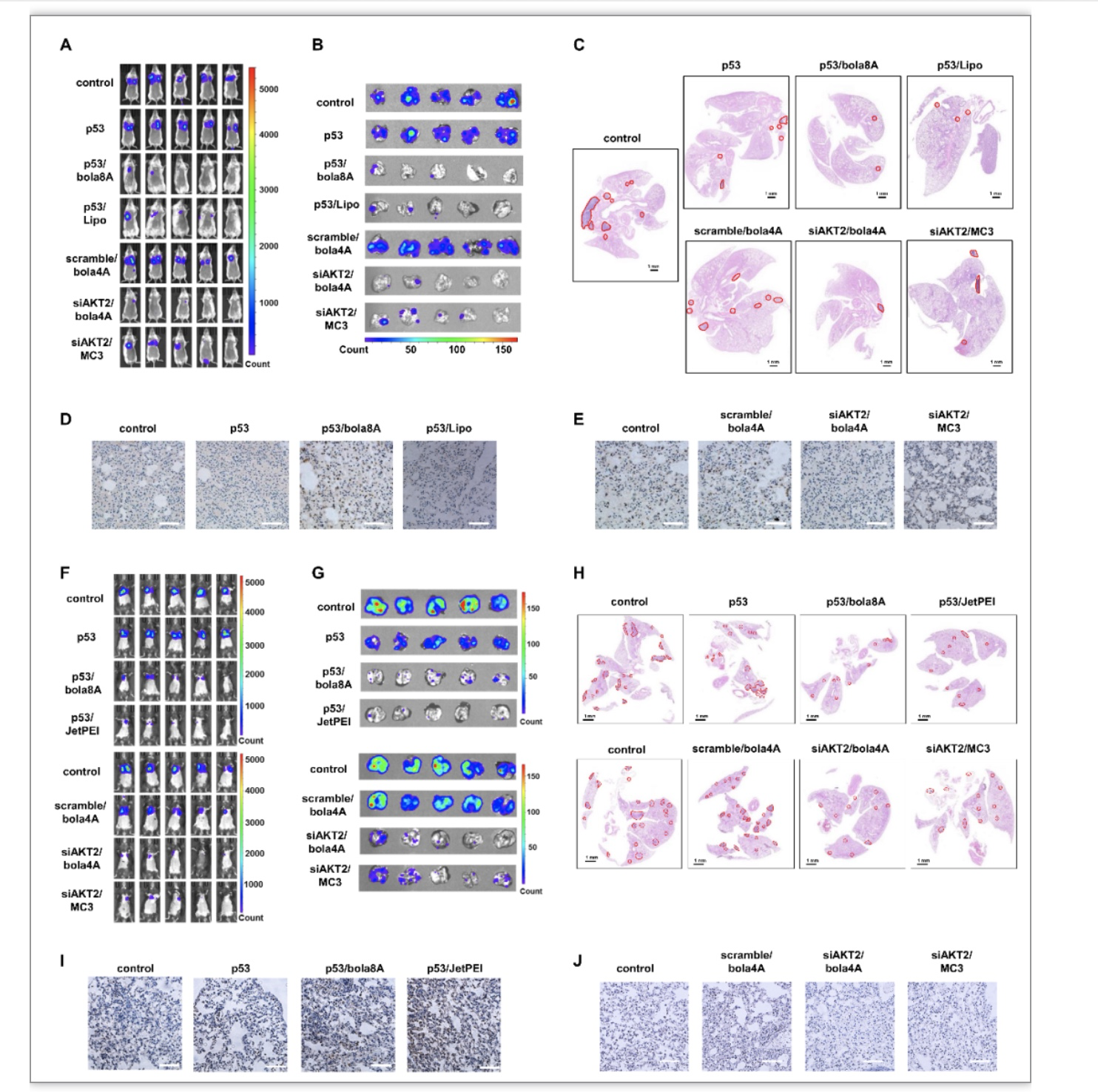
Fig. 3. Effective inhibition of tumor metastasis using DNA and siRNA therapeutics delivered by bola8A and bola4A respectively in lung metastatic cancer model. (A-E) 4T1-luc metastatic tumor-bearing mice received intravenous injections of PBS buffer (control), p53 alone, p53/bola8A complex, p53/Lipo complex (2.0 mg/kg DNA, 1.5 mg/kg bola8A, N/P ratio of 1.0), siAKT2 alone, siAKT2/bola4A complex, or siAKT2/MC3 complex (1.0 mg/kg siRNA, 3.9 mg/kg bola4A, N/P ratio of 5.0) (n=5): (A) In vivo bioluminescence imaging of 4T1-luc tumor metastases in the mice. (B) Ex vivo bioluminescence imaging of 4T1-luc tumor metastases in the lung at the experimental end point post treatment. (C) Histological analysis of lung tissues from 4T1-luc metastatic tumor-bearing mice at the experimental end point post treatment. The metastatic lesions (red solid outlines) were identified as cell clusters with darkly stained nuclei. Scale bars, 1 mm. (D) p53 and (E) AKT2 protein expression revealed by immunohistochemistry staining after treatments. Scale bar, 200 μm. (F-J) B16-F10-luc metastatic tumor-bearing mice received intravenous injections of PBS buffer (control), p53 alone, p53/bola8A complex, p53/JetPEI complex (2.0 mg/kg DNA, 1.5 mg/kg bola8A, N/P ratio of 1.0), siAKT2 alone, siAKT2/bola4A complex, or siAKT2/MC3 complex (1.0 mg/kg siRNA, 3.9 mg/kg bola4A, N/P ratio of 5.0) (n=5): (F) in vivo bioluminescence imaging of B16-F10-luc tumor metastases in the mice. (G) Ex vivo bioluminescence imaging of B16-F10-luc tumor metastases in the lung tissue or images of excised lung tissues at the experimental end point post treatment. (H) Histological analysis of lung tissues from B16-F10-luc metastatic tumor-bearing mice at the experimental end point post treatment. The metastatic lesions (red solid outline) were identified as cell clusters with darkly stained nuclei. Scale bars, 1.0 mm. (I) p53 and (J) AKT2 protein expression revealed by immunohistochemistry staining after treatments. Scale bar, 200 μm. p53: plasmid DNA expressing tumor suppressor protein p53, siAKT2: siRNA targeting AKT2.
目前获批上市的核酸药物载体-脂质纳米粒(lipid nanoparticles, LNPs)-通常由多组分脂质构成,制剂稳定性较低,且存在安全性隐患。此外,针对不同类型核酸药物的递送仍需重新进行制剂处方筛选。由于不同类型的核酸药物的性质(大小、尺寸、构象等)千差万别、作用位点和机制各不相同,因此迫切需要开发一类可按需定制、安全、高效,且适用于不同类型核酸递送的载体平台技术。