背景
驱动蛋白是一类分子马达,它利用ATP水解的能量沿着微管细丝的表面移动或破坏微管细丝的稳定。驱动蛋白马达在细胞分裂、细胞运动、细胞内运输和纤毛功能中起着重要作用,对其机制和功能的了解已经取得了很大进展。关于在细胞中如何调节驱动蛋白以确保其基于微管的活动的时间和空间问题尚不清楚。
图1. 驱动蛋白(图片来源于网络)
驱动蛋白超家族的所有成员都包含一个驱动蛋白运动结构域(浅绿色),可以位于多肽链的两端或中间。一般来说,运动结构域在多肽序列中的位置表明了蛋白质的定向运动性。具有氨基末端运动结构域的驱动蛋白向微管的正端运动(快速生长),而含有羧基末端运动结构域的驱动蛋白则相反,向微管的负端(缓慢生长)运动。许多驱动蛋白构包含盘绕的线圈段(深绿色)用于寡聚化。另外,驱动蛋白包含独特的非运动结构域(蓝色),赋予不同驱动蛋白家族特定的调控和功能特性。例如,具有中心运动结构域的Kinesin - 13家族不进行定向运动,而是在其正负端破坏微管的稳定。kinesin - 8和kinesin - 14家族可以行走并破坏微管的稳定等。
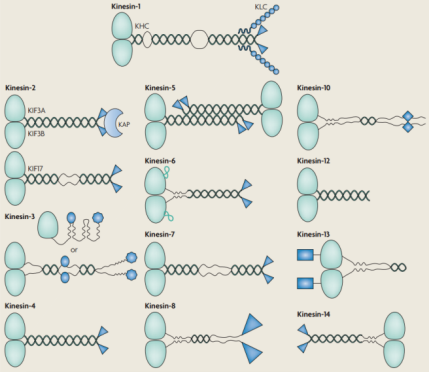
图2. 驱动蛋白家族
目前已在人类中发现了45个具有不同功能的KIFs,根据氨基酸序列和结构差异,它们被分为14个亚家族(Kinesin 1-14)。大多数驱动蛋白以同型二聚体存在(例如,kinesin-4、kinesin-6、kinesin-7、kinesin -8、kinesin-10、kinesin-12、kinesin-13和kinesin-14)。
表1. 驱动蛋白家族及其功能
|
Family |
Functions |
commonly studied family members |
inhibitor |
|
kinesin-1 |
Vesicle, organelle and mRNA transport |
• KIF5 (Mm), KHC (Dm and Nc) and UNC-116 (Ce) |
- |
|
kinesin-2 |
Vesicle, melanosome and intraflagellar transport |
•Heterotrimeric subfamily: KIF3 (Mm), KLP64D(Dm), KLP68D (Dm), KRP85 (Ce), KRP95 (Ce), Kin1 (Tt), Kin2 (Tt) and FLA10 (Cr) • Homodimeric subfamily: KIF17 (Hs), OSM-3 (Ce) and Kin5 (Tt) |
- |
|
kinesin-3 |
Vesicle transport |
• Subfamily: KIF1 (Hs), UNC104 (Dm), UNC-104 (Ce) and Kin3 (Nc) • Subfamily: KIF13 (also known as GAKIN; Hs), KIN73 (also known as KHC73;Dm) and KLP-4 (Ce) • Subfamily: KIF28 (Hs) and KLP-6 (Ce) • Subfamily: KIF16 (Hs) and KLP98A(Dm) • Subfamily: KIF14 (Hs) |
- |
|
kinesin-4 |
Chromosome positioning |
• KIF4 (Hs), chromokinesin (Gg), KLP3A(Dm) and KLP1 (also known as KIF4; Xl) |
- |
|
kinesin-5 |
Spindle pole separation and spindle bipolarity |
KIF11 (also known as Eg5; Hs), KIF11 (Mm), Eg5 (Xl), KLP61F (Dm), BimC(An)BMK-1 (Ce), Cin8 (Sc), Kip1 (Sc) and Cut7 (Sp) |
ARQ621、Dimethylenastron、S-Tritylcysteine、K858、PVZB-1194、Litronesib (LY2523355)、BRD9876、MK-0731、ARRY-520、Ispinesib (SB-715992) |
|
Kinesin-6 |
Central spindle assembly and cytokinesis |
• Subfamily: MKLP2 (also known as KIF20A;Hs), KIF20A (also known as Rab6 kinesin; Mm) and Subito (Dm) • Subfamily: MKLP1 (also known as KIF23;Hs), KIF23 (Mm), CHO1 (Cg), Pavarotti (Dm) and ZEN-4 (Ce) |
Paprotrain |
|
Kinesin-7 |
Kinetochore–microtubule attachment and chromosome congression |
• KIF10 (also known as CENPE;Hs), KIF10 (Mm) and KIP2 (Sc) |
GSK923295、PF-2771 |
|
Kinesin-8 |
Chromosome congression |
• Subfamily: KIF18 (Hs) and KLP67A(Dm) • Subfamily: KIF19 (Hs), KLP13 (Ce), KIP3 (Sc), KLP5 (Sp) and KLP6 (Sp) |
AMG650、BTB-1 |
|
Kinesin-9 |
intraflagellar transport |
Subfamily: kinesin-9A(also known as KIF9);kinesin-9B(also known as KIF6) |
- |
|
Kinesin-10 |
Chromosome positioning |
KIF22 (also known as KID; Hs and Xl) and Nod (Dm) |
- |
|
Kinesin-12 |
Spindle pole organization |
• KIF12 (Mm), KIF15 (Mm), KLP54D(Dm) and KLP2 (also known as KIF15A; Xl) |
GW406108X (GW108X)、Kif15-IN-2 |
|
Kinesin-13 |
Kinetochore–microtubule error correction and chromosome segregation |
• Subfamily: KIF2A(Hs), KIF2B (Hs), MCAK (also known as KIF2C;Hs), MCAK (Cg), KCM1 (also known as KIF2C; Xl), KLP10A(Dm), KLP59C(Dm), KLP59D(Dm) and KLP7 (Ce) • Subfamily: KIF24 (Hs) and Kin1 (Pf) |
- |
|
Kinesin-14 |
Spindle pole organization and cargo transport |
• Subfamily: KIFC1 (also known as HSET;Hs), CHO2 (Cg), NCD(Dm), CTK2 (Xl) and Kar3 (Sc) • Subfamily: KIFC2 (Hs), KIFC3 (Hs), KLP3 (Ce) and a large number of plant-specific isoforms An, Aspergillus nidulans; Ce, Caenorhabditis elegans; Cg, Cricetulus griseus; |
SR31527 chloride、CW-069、AZ82 |
kinesin-8驱动蛋白是微管动力学和组织的调节剂,在细胞分裂过程中,微管对细胞内运输、细胞运动、纺锤体组装和染色体分离至关重要。Kinesin-8家族成员包括 KIF18, KLP67A, KIF19, KLP13, KIP3, KLP5和KLP6。它们可以运动到微管正端,当它们到达微管顶端时,通过去除微管蛋白亚基来破坏GTP的稳定性。它们在较长微管的尖端积聚的水平更高,解聚微管的效率也高于较短的微管。kinesin - 8的某些成员也可以稳定微管和“抑制”微管动力学,这对于调节附着在着丝点微管纤维上的着丝点的运动很重要。
KIF19
KIF19是一种参与细胞内物质运输的驱动蛋白,其在细胞分裂、细胞形态维持、物质运输等多种细胞生物学过程中发挥重要作用。关于KIF19更详细的作用机制和功能还需进一步的深入研究和了解。
优爱可提供KIF19蛋白,用于药物筛选与验证,助力相关药物研发。
★ 蛋白纯度高(>85%);
★ 经验证的高活性
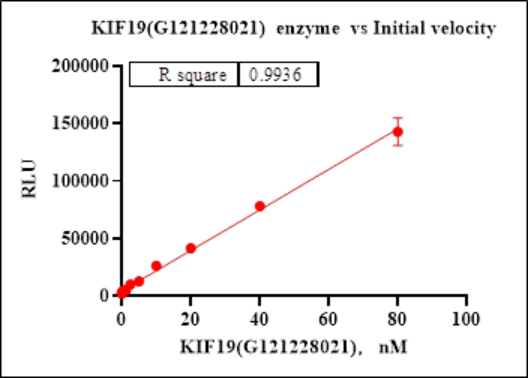
图3. KIF19蛋白活性验证
参考文献:
1.Punekar SR, Velcheti V, Neel BG, Wong KK. The current state of the art and future trends in RAS-targeted cancer therapies. Nat Rev Clin Oncol. 2022 Oct;19(10):637-655.
2.Tomei EJ, Wolniak SM. Kinesin-2 and kinesin-9 have atypical functions during ciliogenesis in the male gametophyte of Marsilea vestita. BMC Cell Biol. 2016 Jul 16;17(1):29.
3.Cross RA, McAinsh A. Prime movers: the mechanochemistry of mitotic kinesins. Nat Rev Mol Cell Biol. 2014 Apr;15(4):257-71.
4.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015 Dec;16(12):711-26.
来源于优宁维药物研发官网